Culver City-based Avenda Health received the green light from the Food and Drug Administration last month for iQuest, a patient management software that identifies and visualizes prostate cancer.
The artificial intelligence-enabled iQuest differentiates itself through patient-specific diagnostic information and deep-learning algorithms. The technology creates a map pinpointing the exact location of prostate cancer in a patient, enabling personalized and precise treatments.
The problem iQuest resolves, according to Avenda, is the one-size-fits-all approach of treating the entire prostate. The method is used because current MRI technology cannot identify the full extent of the tumor and cancer growth within the prostate, which, according to Avenda, results in nearly 50% of patients losing their sexual or urinary functions.
What’s more, existing artificial intelligence, or AI, products are primarily used in diagnosis as opposed to treatment, via digital radiology or digital pathology.
“It is aimed towards urologists for use after a patient already has a diagnosis of prostate cancer (and a Gleason Grade) in order to help the physician decide what to do next,” Dr. Shyam Natarajan, co-founder and chief executive of Avenda Health, said, adding that MRI alone underpredicts the extent of disease. Gleason grades help with understanding the prognosis of men with prostate cancer.
The iQuest platform provides physicians with a 3D visualization of the cancer and offers a better understanding of the extent of the disease to aid in treatment planning, which can better preserve quality of life while minimizing the cancer left behind after surgery or other procedures.
“We are excited about the potential to unlock precision care in prostate cancer with iQuest, as it is a key enabling technology for focal therapy to be a reality for urologists and patients,” Natarajan said.
“In order for a doctor to treat focally, they need to know where cancer is and the healthy tissue to avoid. This is vital information that iQuest now provides. This is a huge step forward in transforming the standard of care in prostate cancer and brings us that much closer to offering effective therapy that preserves quality of life to providers and patients across the United States.”
Decade of research
In addition to iQuest enabling physicians to pinpoint the cancer’s location and severity as opposed to treating the entire organ, patients will benefit from treatment selection, planning, guidance and follow-up evaluations.
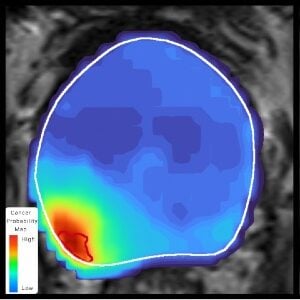
Building the platform was no easy feat, as iQuest now sits upon nearly a decade of research from hundreds of thousands of data points and multiple clinical studies. One of the iQuest studies resulted in urologists improving their efficiency of identifying tumor extent from 37% to 97%.
Avenda was spun out of UCLA five years ago. Ensuring that it had a suitable regulatory path was one of the endeavor’s key challenges, according to Natarajan. The company now has 18 full-time employees, including experts in AI, urology and imaging.
“AI and software is quite new at the FDA, especially products that explicitly aid in clinical decision making,” Natarajan wrote. “Thankfully, after multiple meetings with the FDA we were able to identify and then validate our platform with a clear path — ending up with the clearance that we just obtained.”
The enabling technology for iQuest is known as fusion biopsy, a technique in which MRI and ultrasound are merged to better guide urologists in making accurate diagnoses.
Avenda has made iQuest compatible with multiple treatment options including FocalPoint, the company’s own soft tissue-laser ablation device. FocalPoint can treat prostate cancer in an office setting under ultrasound guidance.
The potential market for iQuest is a large one, considering that about one out of eight men will be diagnosed with prostate cancer during their lifetime, according to the American Cancer Society. About six of 10 cases are diagnosed in men who are 65 or older, and the average age at diagnosis is about 66.
Available soon?
As far as turning a profit from the technology, Natarajan wrote that Avenda is partnering with institutions in a beta period but anticipates that it will be available to patients shortly.
“At the heart of what we do is the belief that patients should never have to choose between quality of life and cancer control,” Dr. Brittany Berry-Pusey, co-founder and chief operating officer of Avenda Health, said. “By providing a personalized cancer map, iQuest opens the door for more precise treatments. This clearance will go a long way not only for our company, but for the future of prostate cancer care.”
Avenda is a little more than four months past its series B funding round, which netted $10 million in a fundraise led by VCapital. The Chicago-based firm’s exit technology portfolio consists of Similarity Machines, Cleversafe and Imagineer Technology Group. VCapital invested into Cleversafe in 2005, and the company was later acquired by IBM in 2015.
The capital, which brought Avenda’s total funding to $16 million last August, is being used to accelerate the use of iQuest and to continue clinical evidence development. The round also included participation from Plug & Play Ventures and Wealthing VC Club.
“Our company’s mission is to provide clinicians and their patients greater access to care while maintaining quality of life that is often missing in prostate cancer treatment. Our technology is solving key issues in men’s health, and we look forward to creating real change in prostate cancer care,” Natarajan said in a statement. “This funding will play a critical role in expanding the capability and reach of our technology, while adding to our experienced team of urology, medical device, and AI leaders.”
