Culver City-based Avenda Health, which has developed a laser heat treatment platform targeting prostate cancer and related conditions, had two key developments last month.
On Aug. 9, the company announced the U.S. Food and Drug Administration had given clearance for a randomized clinical trial of its laser ablation technology to treat prostate cancer.
Then, on Aug. 25, the company announced it had raised $10 million in Series B funding led by Chicago-based VCapital, a venture capital firm.
Both are key stepping stones on the way to commercializing laser heat therapy to treat prostate cancer.
Avenda Health was started by three UCLA faculty colleagues in June 2017; two of the co-founders – Brittany Berry-Pusey and Chief Executive Shyam Natarajan – were co-founders of the UCLA Business of Science Center launched by the late businessman and innovator Roy Doumani. The third co-founder, Chief Medical Officer Leonard Marks, is a urology professor at the UCLA David Geffen School of Medicine.
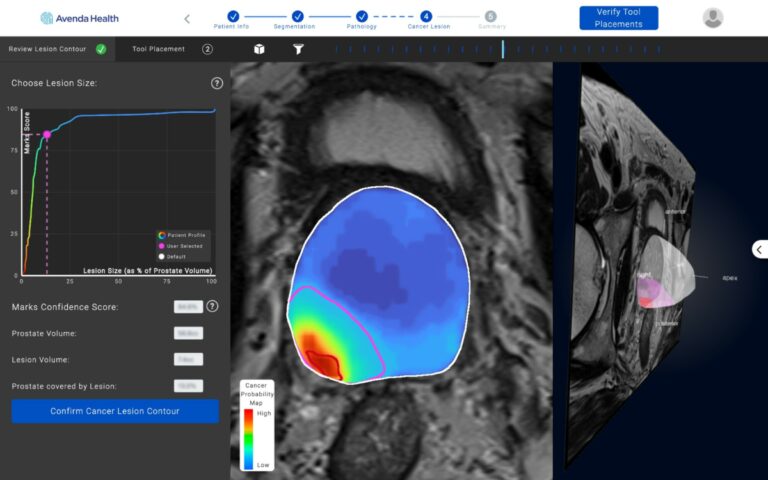
The trio sought to develop laser ablation technology to treat prostate cancer that could be applied in an outpatient setting. The technology involves the use of a laser needle and thermal optical sensor to precisely target and treat soft tissue. The added precision minimizes urinary incontinence and sexual dysfunction, two common side effects of conventional radiation or chemotherapy treatments for prostate cancer.
In developing the laser system, Avenda found that more accurate mapping of the target prostate cancers was needed. They developed a 3D mapping system that uses artificial intelligence algorithms and probabilities to create a map of the cancer zone that can then be used to guide treatment. Both can be used in an outpatient setting.
The FDA clearance involved the issuance of an investigational device exemption to allow these two technologies to be used together in a randomized control trial. The goal is to demonstrate a higher success rate with fewer harmful side effects in treating prostate cancer than more traditional chemotherapy or surgical techniques.
“Our mission is to advance prostate cancer therapy so patients no longer need to choose between treatment or quality of life,” Natarajan said in the company’s announcement.
“Using the latest deep learning technology, iQuest gives physicians and their patients more insights to identify the best treatment on an individual basis,” Natarajan added. “We’re thrilled to receive IDE approval so we can further prostate cancer research for the millions of men affected each year.”
According to the announcement, if the clinical trial proves successful, it could lead to the first FDA approval of a localized treatment therapy for prostate cancer in more than four decades.
Last year, Avenda received a breakthrough device designation from the FDA for its laser ablation system; that designation signifies the agency believes there is a high likelihood the technology will be better than the current standard of care.
The $10 million fundraising round Avenda announced on August 25 will help the company complete the randomized clinical trial.
VCapital partner Ryan Kole said in the announcement that Avenda’s focus on improving men’s health is part of the reason for taking on the lead role in the funding round.
“We are thrilled to be a part of Avenda Health’s journey in making incredible strides in the future of prostate cancer care,” Kole said. “Investing in something meaningful, innovative and groundbreaking in men’s health, is something VCapital is proud of.”
The company said the $10 million in new capital would be used to expand the use of its 3D mapping platform and continue clinical evidence development. It follows approximately $9 million in funds the company had previously raised, bringing the total to $19.3 million.